Start Technology & Products TRANBERG system (uro)
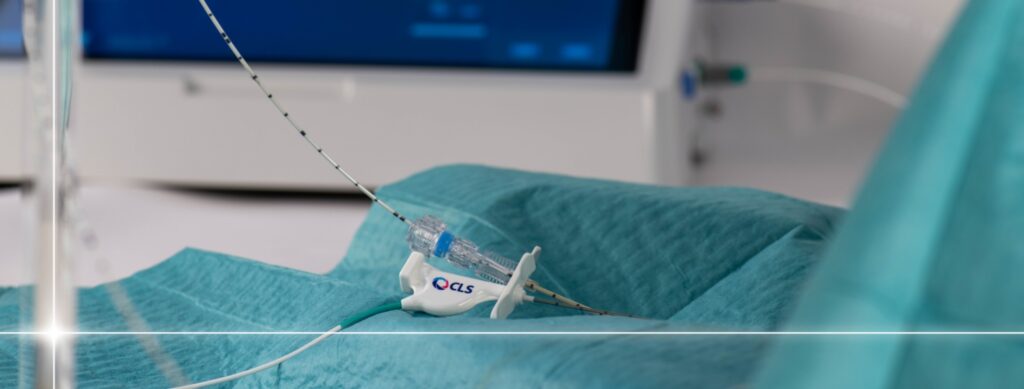
Focal laser ablation (FLA) is an effective way to precisely target and destroy benign and malignant tumors and other pathological tissue in a minimal invasive setting. The TRANBERG® Thermal Therapy System is based on patented technology and developed and manufactured by CLS.
The TRANBERG system is CE-marked* (EU) and 510(k) cleared by the FDA** (the US) and mainly used in urology with focus on localized prostate cancer.
System Brand Name TRANBERG® Thermal Therapy System
Legal Manufacturer Clinical Laserthermia systems AB (CLS)
Market Approvals CE marked and FDA 510(k) cleared
Image Guidance MRI or MRI US-fusion
Distributed by Tecnogamma SRL (Italy) and MTEC Company (France)
*Intended purpose, EU (CE, MDR)
The Mobile Laser Unit is intended for thermal ablation of soft tissue pathological lesions.
The Laser Applicator Non-cooled is intended to deliver laser energy from the Mobile Laser Unit to the targeted soft tissue pathological lesion, resulting in thermal ablation of the lesion. The Laser Applicator Non-cooled is used together with the Mobile Laser Unit.
The MR Introducer is intended as an aid for the insertion of the Laser Applicator Non-cooled into the targeted soft tissue pathological lesions.
The Tissue Temp Probe can be used together with the Mobile Laser Unit and the Laser Applicator Non-cooled. The Tissue Temp Probe is intended for tissue temperature monitoring in manual mode or for tissue temperature regulation in feedback mode. The Tissue Temp Probe is used according to indications of the Laser Applicator Non-cooled.
All devices above are intended to be used by trained medical health care professionals in hospitals or in outpatient clinics.
Thermoguide Workstation is intended to retrieve, store, process, and display temporally dynamic magnetic resonance (MR) data from scanners to monitor temperature changes. When used to monitor thermal ablation, the software can also calculate thermal damage induced by ablation devices. When connected to compatible laser unit it allows the user to control the laser output from the user interface.
**Intended use / Indications for Use, the US (FDA 510(k))
The TRANBERG®|Thermal Therapy System is indicated for use in surgical applications requiring the ablation, vaporization, excision, incision, and coagulation of soft tissue in areas of surgery including: gastroenterology, general surgery, plastic surgery, genitourinary (urology), gynecology (GYN), neurosurgery, otolaryngology (ENT) head and neck, orthopedics, ophthalmology, pulmonology, and thoracic surgery.
–
Italy Tecnogamma SRL >>
France MTEC Company >>
Welcome to Clinical Laserthermia Systems.
Please note that all our products and indications are not yet approved in all markets. Don’t hesitate to contact us for up-to-date market approvals in your area!
Please choose your country or region