Start Technology & Products Technology Platform

Our patented thermotherapy platform includes innovative mobile laser devices, temperature monitoring devices, and sterile disposables, and is commercialized as regulatory-approved products through strategic partners in the U.S. and Europe, with a vision to do so on a global scale.
Laser ablation is an effective way to destruct benign and malignant tumors and other pathological tissue in a minimally invasive setting. The CLS product portfolio is based on patented technology and designed to deliver laser energy to heat the target tissue with high precision. During a treatment session, tissue temperature and necrosis of the target tissue, can be controlled by the user with high accuracy, while saving surrounding healthy tissue.


The mobile laser unit is the laser source and controlling component of our systems.
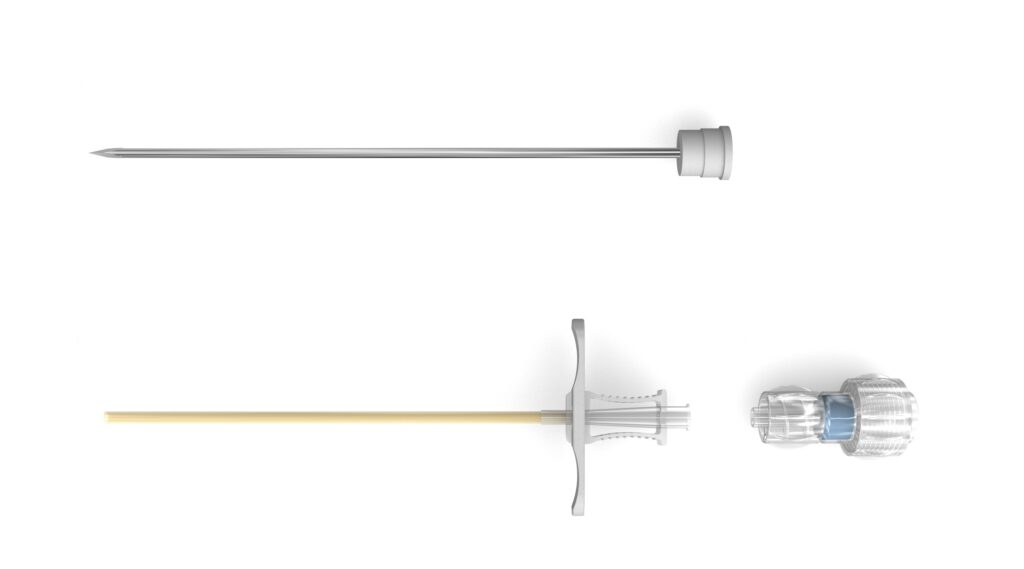
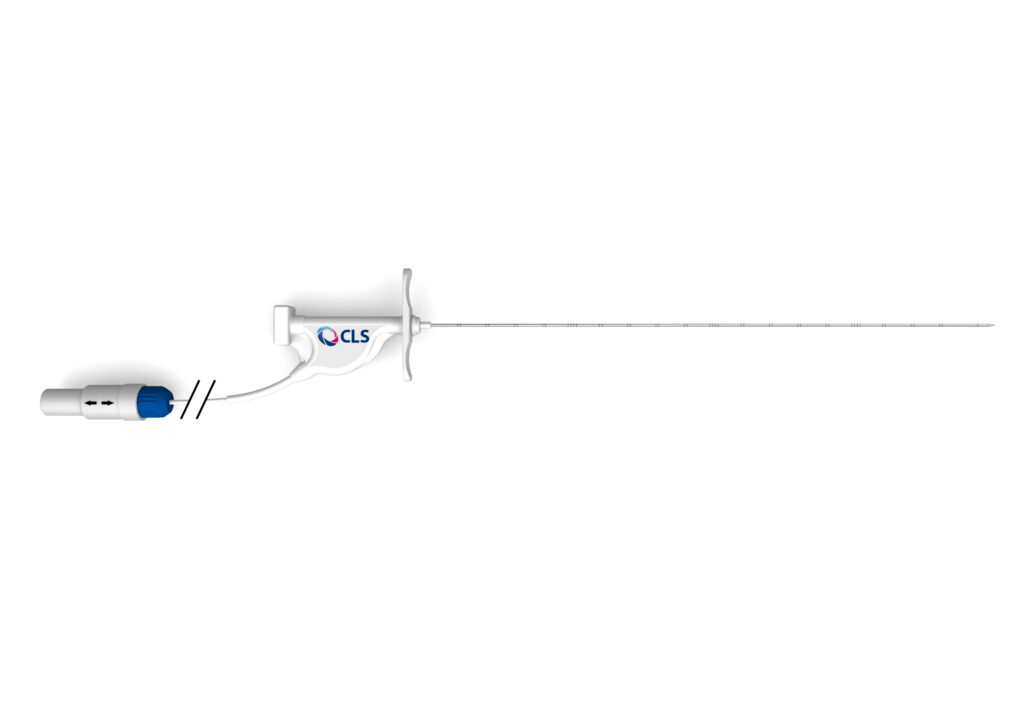
Non-cooled laser applicators, and procedure specific Introducer systems designed to suit anatomical needs.
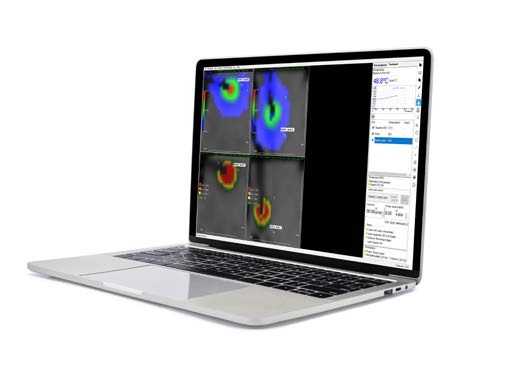
Temperature monitoring devices and safety guards enabling the physician to follow the progression of the ablation in near real-time.
At CLS, we are an agile, focused organization dedicated to turning breakthrough product innovation and design to the clinic.
We concentrate our resources on our core strengths:
We seek partners who:
Welcome to Clinical Laserthermia Systems.
Please note that all our products and indications are not yet approved in all markets. Don’t hesitate to contact us for up-to-date market approvals in your area!
Please choose your country or region