Start Technology & Products Prism system (neuro)

When using laser ablation in neurosurgery, so-called laser interstitial thermal therapy (LITT), a region of interest is directly targeted and treated with high precision. The unique and patented Prism system is used for various diseases and conditions affecting the brain, especially when the target area is hard-to-reach, or the patient is ineligible for open surgery*.
The Prism system is developed and manufactured by CLS, and commercialized in the US by our partner ClearPoint Neuro Inc. The use of the Prism system in combination with the neuro navigation access systems of ClearPoint Neuro ensures ease of use, high accuracy and workflow efficiency.
System Brand Name ClearPoint Prism® Neuro Laser Therapy System
Legal Manufacturer Clinical Laserthermia systems AB (CLS)
Market Approval FDA 510(k) cleared (The U.S.)
Image Guidance MRI
Distributed by ClearPoint Neuro Inc.
*Intended use / Indications for Use, the U.S. (FDA 510(k))
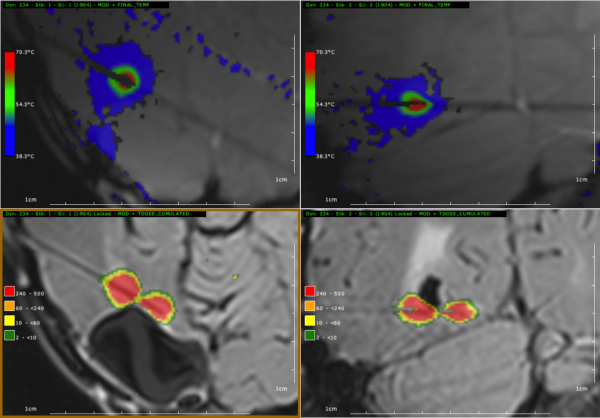
The ClearPoint Prism®|Thermoguide Therapy System is indicated for use to necrotize or coagulate soft tissue through interstitial irradiation or thermal therapy under magnetic resonance imaging (MRI) guidance in medicine and surgery in neurosurgery, for a wavelength of 1064nm.
When therapy is performed under MRI guidance, and when data from compatible MRI sequences is available, the ClearPoint Prism®|Thermoguide therapy system can process images using proton resonance-frequency (PRF) shift analysis and image subtraction to relate changes in complex phase angle back to relative changes in tissue temperature during therapy. The image data may be manipulated and viewed in a number of different ways, and the values of data at certain selected points may be monitored and/or displayed over time.
The ClearPoint Prism®|Thermoguide Therapy System is compatible with 3.0T MR scanner systems: Siemens MRI Magnetom and GE MRI Signa. When interpreted by a trained physician, this device provides information that may be useful in the determination or assessment of thermal therapy. Patient management decisions should not be made solely on the basis of analysis using the ClearPoint Prism®|Thermoguide Therapy System.
Welcome to Clinical Laserthermia Systems.
Please note that all our products and indications are not yet approved in all markets. Don’t hesitate to contact us for up-to-date market approvals in your area!
Please choose your country or region